Unconventional. Restless. Disruptive.
Clinical trial management reinvented
Elixia is a trusted clinical site organization for Phase I – IV trials, providing industry-leading patient recruitment and clinical trials management and analysis for Cardiology/Nephrology, Psychiatry/Neurology, Complex Metabolic and Infectious Disease – not just for a single trial, but across your entire therapeutic pipeline.
Unconventional. Restless. Disruptive.
Clinical trial management reinvented
Elixia is a trusted clinical site organization, providing industry-leading patient recruitment and clinical trials management and analysis for Cardiology/Nephrology, Behavioral Health and Infectious Disease – not just for a single trial, but across your entire therapeutic pipeline.

Elixia Clinical Research:
Services and Capabilities Brochure
Our breakthrough approach helps you meet milestones with speed and agility, and we enroll and retain the right patients, trial after trial.

Elixia Clinical Research:
Services and Capabilities Brochure
Our breakthrough approach helps you meet milestones with speed and agility, and we enroll and retain the right patients, trial after trial.
Partnering across your portfolio to…
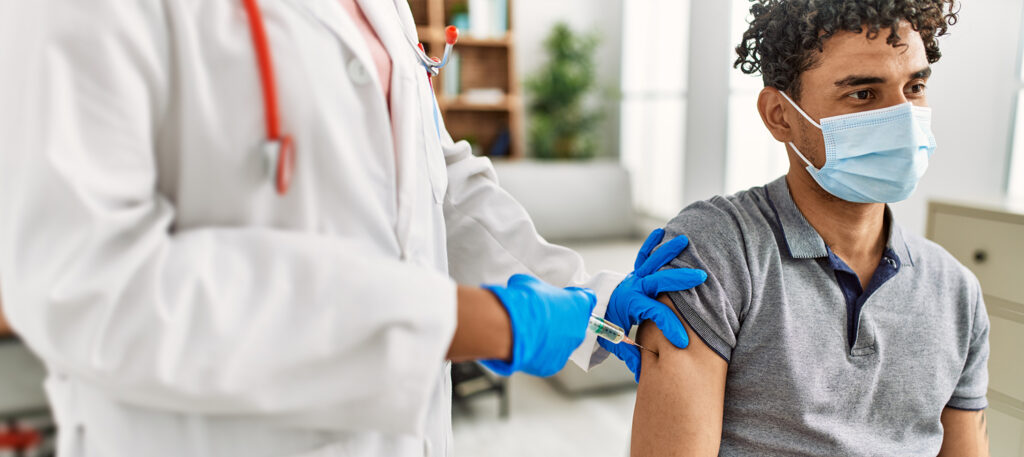






Reinvented processes for repeated successes
STUDY STARTUP AND REGULATORY SUBMISSION
3-4 Days
FIRST RANDOMIZED PATIENT POST SITE ACTIVATION
1 – 2 Days
50% OF INITIAL ENROLLMENT GOAL, ON AVERAGE
12-16 Weeks
HOW WE DO IT
TECHNOLOGY
Expedite trials with our ElixiaXscientia advanced technology platform.
Rapid Patient Enrollment
Focus on Diversity and Inclusion
UNCOMMON THERAPEUTIC EXPERTISE
Our extensive national network represents deep therapeutic expertise in highly specialized areas

Cardio-Renal Network
Behavioral Health

Infectious Disease
REFINED MANAGEMENT PROCESSES
We eliminate traditional trial bottlenecks with a singular combination of specialized clinical expertise and an agile operating model with reproducible processes to deliver unprecedented results.

Reproducible Excellence
Curiosity and Perspective
Disrupting the one-and-done paradigm with pipeline strategies for your success from early phase through commercialization